Ginsenoside PPD found more potent against cancer cells than Rg3 and Rh2
Ginseng is one of the most popular herbs around the world. Traditionally originating in East Asia, ginseng has gained huge popularity among western people since it was introduced to western countries.
People love ginseng because it has many health benefits. Ginseng could help:
- boost energy
- enhance physical performance
- support cognition
- regulate immunity
- prevent mental stress
- enhance healthy glucose levels
The medicinal benefits of ginseng are derived from pharmacologically active compounds, called ginsenosides. To date, about 100 ginsenosides have been isolated and identified. Some well-known ginsenosides include Rb1, Rb2, Rg3, Rh2, and Rg1, Rg2, and Rh1.
Ginsenoside Rg3 and Rh2 are well-known ginsenosides for their early practical use in promoting human health. For example, ginsenoside Rg3 is already listed as a new anticancer drug in traditional Chinese medicine in China and widely used in the treatment of different types of cancer.
The research in ginsenosides has made progress in recent years, and scientists have found more bioactive ginsenosides than ginsenoside Rg3, and ginsenoside PPD, also known as aPPD, is a good case.
A recent study published in the journal Cell Death & Disease found the antitumor effects of ginsenoside PPD and its possible mechanisms. One finding of the study is that ginsenoside PPD has the best antitumor activities than other four ginsenosides, Rh2, Rg3, Rg5 and Rh3.
In the study, scientists evaluated the antitumor activity of five ginsenosides, namely PPD, Rg5, Rg3, Rh2 and Rh3 in five different cancer cell lines (PLC/ PRF/5 human liver hepatoma cells, PANC-1 human pancreatic carcinoma cells, A549 human lung carcinoma cells, MCF-7 breast cancer cells, and HCT-8 human colon carcinoma cells respectively).
The study result showed that all five ginsenoside monomers showed inhibitory effects on the proliferation and migration of each tumor cell line in a time-dependent manner.
Typically, the half-maximal inhibitory concentration (IC50) of ginsenoside PPD was about 70 µM, lower than that of other monomers, which means that ginsenoside PPD had the best inhibitory effects on the cell viability of each cancer cell line.
Similarly, when it comes to the migration inhibition test, ginsenoside PPD also showed the lowest migration rate in all cancer cell lines, and its migration rate was as low as 37% in PLC/ PRF/5 human liver hepatoma cells.
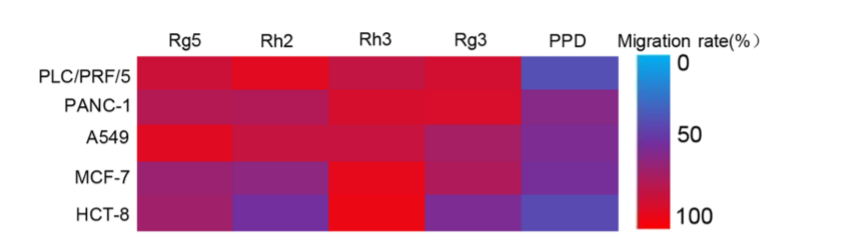
Summary of the migration rates of five ginsenoside monomers for cancer cell lines, PLC/ PRF/5, PANC-1, A549, MCF-7 and HCT-8
The study result suggests that ginsenoside PPD may be a more promising anticancer agent, for it exhibits more potent antitumor activities than Rg3.
A question comes with this finding: why ginsenoside PPD is more bioactive than ginsenoside Rg3, or why ginsenoside Rg3 can stand out among hundreds of previously identified ginsenosides to become an anticancer agent.
To answer this question, we should first understand the difference between prototype ginsenosides and rare ginsenosides.
Prototype ginsenosides are naturally existing saponins in ginseng, such as Rb1, Rb2, Rc, Rd, Re, and Rg1, and they account for 80-90% of the total ginsenoside content in ginseng. And scientists have transformed and metabolized many rare ginsenosides from prototype ginsenosides via unique processing methods.
It was found that, compared with prototype ginsenosides, rare ginsenosides are more bioactive with better membrane permeability and bioavailability due to fewer sugar moieties.
Ginsenosides Rg3, Rh2 and PPD are all rare ginsenosides. Noticeably, ginsenoside PPD is a metabolite of ginsenoside Rg3 or Rh2, so it usually presents better bioactivities, antitumor properties included.
References:
Yang, L., Zhang, X. Y., Li, K., Li, A. P., Yang, W. D., Yang, R., … Yang, C. (2019). Protopanaxadiol inhibits epithelial-mesenchymal transition of hepatocellular carcinoma by targeting STAT3 pathway. Cell death & disease, 10(9), 630. doi:10.1038/s41419-019-1733-8


